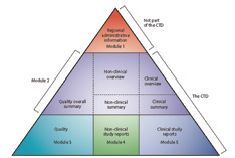
The agreement to assemble all the Quality, Safety and Efficacy information in a common format (called CTD - Common Technical Document ) has revolutionised the regulatory review processes, led to harmonised electronic submission that, in turn, enabled implementation of good review practices. For industries, it has eliminated the need to reformat the information for submission to the different ICH regulatory authorities.
The CTD is organised into five modules. Module 1 is region specific and Modules 2, 3, 4 and 5 are intended to be common for all regions. The CTD became the mandatory format for new drug applications in the EU and Japan in 2003, and for FDA, United States in 2017.
More information: An electronic version of the Common Technical Document (eCTD) can be produced using the information developed by the eCTD Implementation Working Group.